Her medications include metformin, two types of insulin,
metoprolol, Lipitor, Lexapro, and irbesartan. Recently, she has been started on Opdivo (nivolumab) every two
weeks. Within a few months of
starting Opdivo, she developed intense axillary pruritus, and a month or so later, intensively painful fissuring of the fingertips. Each treatment of Opdivo re-exacerbates the fingertip
problem, which is unbearable. Her cancer has gone into remission and she feels well otherwise. She especially enjoys her grandkids.
Her oncologist put her on prednisone 20 mg q.i.d. for the
fingertip eczema. Because of the
patient's diabetes, her fasting blood sugars while on prednisone have gone up over 500
mg%.
EXAMINATION:
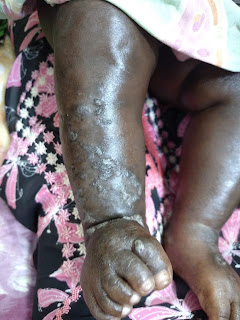
The examination shows erosions on a few of the fingertips without
erythema. Her feet and axillae are
normal.
Clinical Photos:
We did a literature search and there are a few early reports
of dermatitis with Opdivo but they do noot not affect the hands. It may be too early to say; but given the literature on painful hands and feet related to cancer therapies this is
probably a significant reaction.
PLAN: I would
try to keep this simple and avoid the prednisone. Start with wet soak for 20 minutes in warm water, followed
by clobetasol ointment, followed by gloves (a modified Soak and Smear
protocol). We will see how she
does with this. I have the
patient's permission to post her case on this site where we may
get ideas and/or be of help to others with a similar problem. There is a reference to
preventing Hand Foot reactions with chemotherapy. It seems that Celebrex (celecoxib) may be the best option. Note: Celecoxib has been reported to be associated with an increased incidence of heart attack and stroke. It's use should not be cavalier.
References:.
- Survey of cutaneous adverse reactions to targeted cancer therapies: value of dermatological advice. Damiani G OncoSkin working group. G Ital Dermatol Venereol. 2018 May 11. Abstract: From June to October 2012, 25 patients with cutaneous adverse reactions linked to targeted therapies were included in the study. The main prescribed drugs were cetuximab (52%) and erlotinib (20%) and the most common reactions were folliculitis/pustules (40%) and rash/erythema (40%). Hand-foot reaction syndrome was present in 8% of patients. A total of 30% of patients treated for a cutaneous reaction underwent a consultation by a dermatologist. In these patients the rate of oncologic therapy continuation without regimen modifications was higher (100%), while it was progressively lower in patients treated by oncologists (71%) or without any specific treatment (60%). Adverse reaction should be recognized by both dermatologists and oncologists and a multidisciplinary approach is mandatory.
2. Prevention strategies for chemotherapy-induced hand-foot
syndrome: a systematic review and meta-analysis of prospective randomised
trials.
Macedo LT, et.al. Support Care Cancer. 2014
Jun;22(6):1585-93.
Abstract
Amongst 295 studies identified, only ten met the inclusion
criteria. Celecoxib prevented both moderate to severe (odds ratio [OR] 0.39, 95
% confidence interval [CI] 0.20-0.73, P = 0.003) and all-grade HFS (OR 0.47, 95 % CI
0.29-0.78, P = 0.003), whereas pyridoxine and topical urea/lactic acid
formulations failed to prove efficacy. There were no proven benefits in mild
HFS. The use of topical antiperspirant has not been shown to improve results,
according to a single trial.